Methylurea-built SEI unlocks long-life anode-free aqueous zinc batteries
GA, UNITED STATES, December 23, 2025 /EINPresswire.com/ -- A new study introduces a simple molecular-engineering strategy that dramatically improves the reversibility of zinc plating and stripping in aqueous zinc batteries. By using methylurea as a low-cost electrolyte additive, researchers achieved in situ formation of a robust solid electrolyte interphase (SEI) that stabilizes the zinc surface and suppresses parasitic reactions. This engineered SEI enables Coulombic efficiencies approaching 100%, dendrite-free deposition, and unprecedented cycling life across multiple battery configurations. The work demonstrates that a carefully designed interphase can overcome long-standing limitations of aqueous zinc systems and provides a pathway toward practical, cost-effective anode-free zinc batteries with ultrahigh energy efficiency and durability.
Aqueous zinc batteries have attracted widespread attention due to their intrinsic safety, low cost, and high theoretical capacity. Yet their development has been hindered by uncontrollable dendrite growth, low Coulombic efficiency, and severe side reactions during repeated plating and stripping of the zinc anode. These issues are particularly critical for anode-free configurations, which require nearly perfect reversibility to achieve long cycle life. Traditional strategies—including electrolyte engineering, protective coatings, and solvation structure regulation—have offered incremental improvements but still fall short of the efficiency needed for commercial deployment. Due to these challenges, there is a pressing need to develop interfacial chemistries that can stabilize zinc deposition and enable durable anode-free systems.
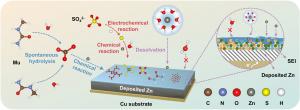
Researchers from the University of Science and Technology of China leading by Prof. Wei Chen (https://staff.ustc.edu.cn/~weichen1) and collaborating institutions reported on August 2025, in eScience, a new electrolyte strategy for stabilizing aqueous zinc batteries. The team developed a methylurea-assisted electrolyte that forms a ZnCO3- and ZnS-rich amorphous solid electrolyte interphase (SEI) directly on the zinc surface, dramatically improving zinc reversibility. The study demonstrates that this interphase design enables long-life anode-free zinc batteries with near-100% Coulombic efficiency and exceptional durability across multiple cathode systems.
Using methylurea as an electrolyte additive, the researchers constructed an optimized SEI layer that fundamentally reshapes zinc deposition behavior. Spectroscopic analysis, NMR, and DFT calculations show that methylurea suppresses water activity and preferentially adsorbs to the zinc surface, initiating strong chemisorption and promoting SEI formation. Microscopy images reveal a uniform ~20 nm amorphous interphase enriched in ZnCO3 and ZnS, which effectively impedes parasitic reactions and eliminates dendritic growth.
Electrochemical evaluation showed remarkable improvements: Cu–Zn asymmetric cells achieved a first-cycle CE of 97.8% and stabilized above 99.9% within 100 cycles, reaching an ACE of 99.93% over 4,500 cycles. Zn–Zn symmetric cells operated for 4,500 hours, while the protective interphase significantly improved corrosion resistance and hydrogen evolution suppression.
Leveraging the highly reversible interphase chemistry, the team assembled multiple anode-free full cells. The anode-free Zn–actived carbon battery delivered 5,000 stable cycles with 80% capacity retention after 3,000 cycles. Additional systems—including high-capacity Zn–I2 and Zn–Br2 configurations—also demonstrated exceptional stability, far outperforming cells using conventional electrolytes. Together, these results affirm methylurea-engineered SEI as a universal and high-performance strategy for aqueous zinc batteries.
"The key to advancing aqueous zinc batteries lies in mastering interfacial chemistry," the leading corresponding author Prof. Wei Chen noted. "Our results show that even trace amounts of a properly designed molecule can completely reshape zinc behavior, enabling efficiencies previously thought unattainable for anode-free systems. By stabilizing the SEI composition at the atomic level, we've demonstrated a practical and scalable pathway for high-performance zinc batteries. This work highlights the power of molecular-level engineering in solving long-standing electrochemical challenges."
The methylurea-enabled SEI strategy offers significant promise for next-generation energy-storage technologies requiring safety, low cost, and long service life. Anode-free zinc batteries could serve in large-scale energy storage, renewable-energy buffering, backup power systems, and portable devices where durability and sustainability are essential. Because the additive is inexpensive, nontoxic, and compatible with low-concentration aqueous electrolytes, the approach is highly scalable for commercial adoption. By overcoming reversibility limitations and eliminating excess zinc, this interphase design accelerates the practical deployment of high-energy aqueous zinc batteries and sets the stage for broader exploration of molecule-driven SEI engineering.
References
DOI
10.1016/j.esci.2025.100397
Original Source URL
https://doi.org/10.1016/j.esci.2025.100397
Funding information
The authors acknowledge funding support from the National Natural Science Foundation of China (52471242, 92372122, 21825302, 22303094) and the Fundamental Research Funds for the Central Universities (WK2060000040, KY2060000150, GG2060127001).
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.